After making two atoms from the website provided in the requirements, here is what I came up with.
The first atom I made was Boron, and it had five protons, five neutrons, and three electrons. The net charge is +2, the mass number is ten. The atomic weight is 10.81 and the density is 2.37 grams per cubic centimeter.
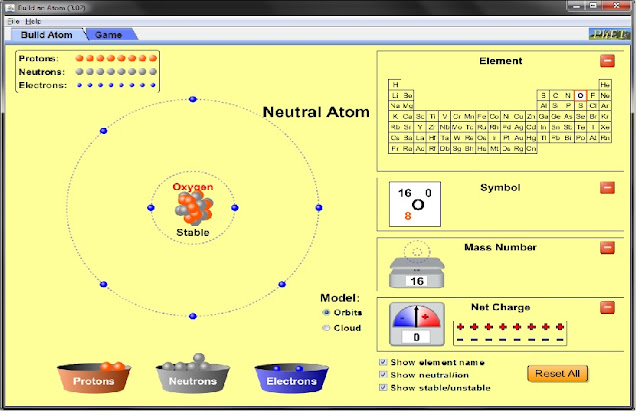
The second atom I made was Oxygen, and it had eight protons, eight neutrons, and eight electrons The net charge is +/- 0, the mass number is 16. The atomic weight is 15.9994 and the density is 1.429 grams per cubic centimeter.
Density = Mass/Volume (g/ml). The simple definition of density: the amount of mass or matter in any given volume.
After completing this I found out that the mass of the ice cube was 4.00 kg, the volume was 4.28 L, and the density of the ice cube is 0.92 kg/l. I actually experimented with all of the possible options from the stimulation, and thought it was very cool to see not only the mass, volume, and density's corresponding but also how much the water shifted every option I clicked.


No comments:
Post a Comment